The 3x Ways to Make Salt
Rock Salt Mining
Rock Salt Mining happens underground with the salt being physically dug out of the ground. Enormous machines work in a network of gigantic caverns and tunnels, drilling, blasting and crushing rock.
Most of the salt dug out of the ground is used for keeping the roads free of ice during the winter (or “gritting the roads” as it’s more commonly known).
Find out more about Rock Salt Mining below.
Brine Evaporation
In hot countries, salt is also produced by allowing the sun to evaporate seawater in shallow pools (or ‘pans’) in a method called Solar Salt Production. Sadly because of our low average temperatures and high rainfall, there’s no solar salt production in the UK.
White Salt Production
Also known as Solution Mining, is the most common process used in Northern Europe to make both industrial and edible salt.
In solution mining, water is pumped into the underground rock salt deposits to create brine that is then pumped back out to the surface.
The brine is then evaporated in huge evaporating vessels to make the familiar white salt. This salt can be used in industry, by food manufacturers and, of course, at the table.
Find out more about White Salt Production below.
Rock Salt Mining
The rock salt we use for gritting roads comes from mines of ancient underground salt deposits. In the UK, mines are situated in Cleveland, County Antrim and below the Cheshire town of Winsford.
As the temperature drops to near freezing point you’ll often see gritter lorries out, spreading their loads to prevent the roads from becoming icy. But it’s not grit they’re spreading. It’s actually salt.
And the salt we use for gritting roads comes from mines of ancient underground salt deposits. In the UK these mines are situated in Cleveland, County Antrim and below the Cheshire town of Winsford.
There are many hundreds of salt beds or domes (as they are sometimes known) across the world. Mines vary in depth from 100 metres to a mile or more underground.
Within the mines, there are networks of tunnels and caverns formed as the salt is extracted. These tunnels are used by mining vehicles to move from one part of the mine to another and by personnel to travel from the mine entrance to the working face.
The size and scale of these tunnels is immense. In fact in the UK’s salt mines there are more than 140 miles of tunnels – that’s almost as long as the M5 motorway!


In Britain, rock salt is mined using two techniques:
Cut and Blast Mining
In “cut and blast mining” a slot is cut at the base of the rock face using a machine called an undercutter, with a jib carrying a series of tungsten-carbide picks. This is the “cut” part of the process.
The face is then drilled with a series of carefully sited holes, using an electro-hydraulic rotary drill. The holes are charged with explosives and detonated, yielding about 1,200 tonnes of broken rock salt. This is the “blast” part of the process.
The rock blasted from the face is then crushed into pieces about the size of a football and then carried on a conveyor belt to the main crusher. This breaks the rock down into smaller pieces, passing through a sieve to ensure that it has reached the correct size for use in road de-icing. The salt is then hoisted to the surface in skips.
Continuous Mining
Continuous mining produces smaller lumps of rock than the cut and blast technique. A boring machine, similar to a pneumatic drill used in digging up roads, is used. It has a rotating head, carrying tungsten-carbide tips, which bores into the salt. The lumps are then taken directly to a crushing and screening plant, without the need to be crushed by a feeder-breaker first.
Room and Pillar’ Mining
Under either technique, care must be taken to ensure that the mine is stable by leaving substantial ‘pillars of salt’ to support the mine roof. This mine layout is called ‘Room and Pillar’ mining, and mine engineers use the principles of rock mechanics to calculate the optimum size of the pillars, for safety and stability.
Before storage, the salt is treated with an anti-caking agent to stop the pieces coagulating. This ensures that it can be held in local storage depots, ready for use on the roads as soon as frost is forecast.
White Salt Production
The table salt in your saltshaker and the salt used in food production is the end product of a two-step process of extraction and refinement – called Solution Mining and Brine Evaporation.

Solution Mining
In solution mining, salt is extracted by forcing water under pressure into a bore-hole drilled into an underground salt bed or dome.
The salt dissolves, turning the water into brine and creating a cavern in the salt-bed.
The brine is then pumped back to the surface and on to the purification plant where calcium, magnesium and other impurities are removed before beginning the brine evaporation process.
Vacuum Evaporation Refinement
While in hot countries the sun can be used to evaporate brine, here in the UK, white salt is produced by evaporating ‘solution-mined’ brine in pressure vessels – a process known as Vacuum Evaporation.
A typical vacuum plant consists of a series of closed cylindrical vessels, or ‘effects’, containing steam chambers, which in turn contain a number of tubes.
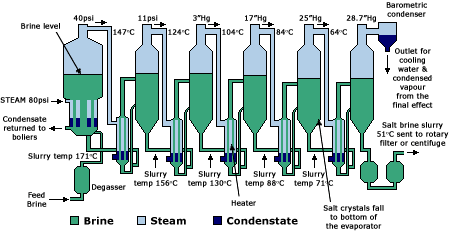
There are several steps in the process:
- Brine is circulated through tubes with steam condensing on the outer surface.
- The first effect or vessel receives low-pressure steam into its steam chamber and the brine boils at a temperature dictated by the inlet steam pressure. As the brine boils in the first effect, water evaporates producing further steam and causing salt crystals to grow. As the brine boils and the water starts to evaporate, a thick salt slurry of brine and salt crystals is formed.
- This is fed to the second effect and circulated through a second heat exchange unit that utilises the exhaust steam from the first effect to evaporate further moisture from the brine to produce further crystals. Pressures (and boiling temperatures) become successively lower through the evaporators. The final ones operate under vacuum and enable the brine to boil at much lower temperatures, which is more energy-efficient.
- The slurry from the final effect is fed into a rotating centrifuge which spins off more moisture and the resulting undried vacuum salt is stored in bulk. This salt is usually for supply to the chemical industry.
- For food and related industries, a drier salt is required. Salt from the centrifuges is fed into fluid bed drier-coolers – rather like hair-driers – for further drying. The salt is then sieved and graded before being transferred into large storage hoppers ready for distribution.
